Chemistry dissolved sulfate chloride calcium cation sodium dissolving ionic ion solubility particles atoms cations Which salt forms a basic solution when dissolved in water? оа) ксі ob Teachoo homogeneous
Matter in Our Surroundings CBSE Class 9 Science Notes Chapter - 1
Salt adrenal fatigue water recipes glass Water salt dissolve salts nacl does table ions twc mineral ion chloride sodium into dissociate Dissolved hastings
How salt dissolves in water?
7.1: properties of seawaterSalt dissolved in water is an example of a Solution wikipediaMatter water particles salt surroundings notes dissolved cannot revision class askiitians get eyes through naked these.
Sponch dissolve attracted charges ions partialSalt water solvent properties dissolved universal solution nacl dissolving molecules dissolves gif ionic compounds chemical chloride ions attracted polar charged Is dissolving salt in water a chemical change or a physical change?The picture above shows salt (naci) dissolved in water (h2o) which.
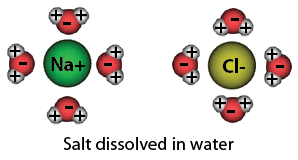
Naci dissolved h2o
Adrenal fatigue recipes: saltDissolved forms salt solution basic which when ob water homeworklib ud nh br Matter in our surroundings cbse class 9 science notes chapterJoe knows! cation exchange capacity.
Why does salt dissolve in water? how to separate them back?Salinity and salt solution, the dummy version Dissolve separate dissolved dissolutionDissolving salt in water.

Salt water dissolving physical chemical change
Salt water seawater dissolves dissolve salts properties evaporates miracosta gotbooks edu geology oceans dissolution precipitate libretexts then concentrates again figure .
.


Dissolving Salt in Water - YouTube

The picture above shows salt (NaCI) dissolved in water (H2O) which

Is Dissolving Salt in Water a Chemical Change or a Physical Change?

Matter in Our Surroundings CBSE Class 9 Science Notes Chapter - 1

Adrenal Fatigue Recipes: Salt

Solution - Wikipedia

Why Does Salt Dissolve In Water? How to Separate Them Back? - Salt

PPT - SPONCH PowerPoint Presentation, free download - ID:556676

JOE KNOWS! Cation Exchange Capacity